Listen to today's episode of StarDate on the web the same day it airs in high-quality streaming audio without any extra ads or announcements. Choose a $8 one-month pass, or listen every day for a year for just $30.
You are here
Chemistry Set
It’s almost a cliché these days, but it’s true nonetheless: We are all made of starstuff. The calcium in our bones, the iron in our blood, and the oxygen in the air we breathe were forged by stars. These elements were expelled into space when the stars died, “seeding” the galaxy with the raw materials for new stars, planets -- and life.
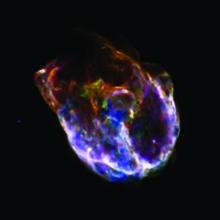
We see many of these ingredients in supernova remnants -- the debris from exploded stars. Sensitive instruments detect dozens of different elements in these rapidly expanding clouds.
There are more of these elements in supernova remnants created by the collapse of massive stars. During their short lives, these stars manufacture a list of heavier elements in their cores, from carbon and oxygen to cobalt and iron. When the core is converted to iron, it can’t continue this process. The core collapses, and the star’s outer layers then blast out into space, ejecting a rich brew of elements.
And the blast itself creates even more elements. The heat and pressure of the explosion causes lighter elements to ram together, forming heavier ones -- especially elements that are more massive than iron.
Debris from such a blast races into space at up to about 10 percent of the speed of light. When the blast wave hits a cloud of cool gas and dust, it can cause the cloud to collapse to form a new star and perhaps planets -- infused with elements forged or released by the death of a heavy star.