Listen to today's episode of StarDate on the web the same day it airs in high-quality streaming audio without any extra ads or announcements. Choose a $8 one-month pass, or listen every day for a year for just $30.
You are here
Cobalt
The electric-vehicle business is facing a bit of bother. A key ingredient in the lithium ion batteries that power electric cars is in short supply. Prices doubled in 2017, and demand is predicted to jump by quite a bit this year.
Cobalt has found many applications over the centuries. It’s been used as a dye for ceramics, and in alloys for jet engines. It’s also a main ingredient in vitamin B12.
Like all but a few of the chemical elements, cobalt was forged in stars. It was made in the hearts of heavy stars, and in powerful stellar explosions.
In the hearts of stars, elements are bombarded with neutrons — the neutral particles in the nuclei of atoms. That builds heavier elements, including a radioactive form of iron. As the iron undergoes radioactive decay, it turns into the stable form of cobalt.
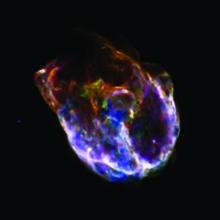
The element also is created when stars explode as supernovae. Such a blast releases enormous amounts of neutrons, which stick to atoms to make heavier elements.
Supernova blasts also make a radioactive form of cobalt. The initial blast produces enormous amounts of a radioactive form of nickel. In a few days, the nickel decays to cobalt. A supernova known as 1987A, for example, produced enough cobalt to make 20,000 planets as heavy as Earth.
But the radioactive cobalt doesn’t last long, either. It decays to a stable form of iron. That process heats the gas expelled by the dead star, keeping it shining brightly for several months.
Script by Damond Benningfield